Since the successful demonstration of a blue light-emitting
diode (LED)1, potential materials for making short-wavelength
LEDs and diode lasers have been attracting increasing interest
as the demands for display, illumination and information storage
grow2–4. Zinc oxide has substantial advantages including large
exciton binding energy, as demonstrated by effi cient excitonic lasing
on optical excitation5,6. Several groups have postulated the use of
p-type ZnO doped with nitrogen, arsenic or phosphorus7–10, and
even p–n junctions11–13. However, the choice of dopant and growth
technique remains controversial and the reliability of p-type ZnO is
still under debate14. If ZnO is ever to produce long-lasting and robust
devices, the quality of epitaxial layers has to be improved as has been
the protocol in other compound semiconductors15. Here we report
high-quality undoped fi lms with electron mobility exceeding that
in the bulk. We have used a new technique to fabricate p-type ZnO
reproducibly. Violet electroluminescence from homostructural
p–i–n junctions is demonstrated at room-temperature.
Extending semiconductor devices to new compounds
has produced great benefi ts to human life, as exemplifi ed by
modern optoelectronic and high-speed electronic devices for
communications attained by GaAs-based III–V compounds16,
and blue LEDs and lasers realized by GaN (ref. 1). Even widerbandgap
compounds such as diamond, AlxGa1–xN, BN (refs 2–4)
and ZnO have attracted considerable interest for applications in
ultraviolet LEDs and lasers and electronic devices durable at highpower
and/or high-temperature operation. Among them, ZnO has
the following advantages for LEDs and lasers. First, the exciton
binding energy in ZnO is as large as 60 meV and can be increased
to over 100 meV in superlattices17. This exciton stability provides
opportunities for making highly effi cient lasers operable at roomtemperature5,6.
Second, it is possible to tune the bandgap from
3 eV to 4.5 eV in MgxZn1–xO and Zn1–xCdxO alloy fi lms with quite
small lattice mismatch between the two different compositions18–20.
This advantage makes it possible to realize strain-free and highquality
quantum wells. Third, large and high-quality single-crystal
wafers are commercially available.
To harvest these advantages in real devices, a reliable technique
for fabricating p-type doping needs to be established. Compared with
other II–VI semiconductors and GaN, it has been diffi cult to dope
ZnO to produce a p-type semiconductor because of a strong selfcompensation
effect arising from the presence of native defects or
hydrogen impurities21,22. The fi rst p-type ZnO was claimed in fi lms
made by vapour-phase transport7 in NH3, followed by molecularbeam
epitaxy (MBE) using an atomic nitrogen source9. These fi lms
had a hole concentration of 1016–1017 cm–3. There are other claims8,10
that As or P doping can achieve hole concentrations higher than
1 × 1018 cm–3. In the former case, it can be naturally understood that
nitrogen replaces oxygen to generate holes because of similar ionic
radius. But the latter cases have raised the following questions14.
The ionic radii of P and As seem to be too large to occupy the oxygen
site within the wurtzite host lattice and thus to serve as acceptors.
Also, from a comparison with established properties of p-type GaN
(ref. 12), such a high concentration of holes would not be expected
in ZnO. None of the studies mentioned addressed signifi cant effort
to growing the high-quality undoped fi lms that generally serve as
a starting point for reliable doping in semiconductors. Here we
propose a repeated temperature modulation (RTM) technique as a
reliable and reproducible way to fabricate p-type ZnO.
Thin fi lms of ZnO and junction devices were grown by laser
MBE using nitrogen as a p-type dopant (see Methods). Figure 1a
shows a typical intensity oscillation of refl ection high-energy
electron diffraction (RHEED) observed during undoped ZnO fi lm
growth at a temperature (Tg) of 950 °C. This layer-by-layer growth
mode has become possible with our development of an atomically
smooth ZnO buffer layer on ScAlMgO4 (SCAM) substrate produced
by high-temperature annealing23,24. The undoped ZnO fi lms
show excellent optical and electronic properties. Figure 1b shows
a photoluminescence spectrum (inset) and temporal variation
of the photoluminescence intensity taken at room-temperature.
The lifetime (τPL) of free-exciton emission reaches 2.5 ns. This τPL is
much longer than the value in ZnO crystal (1 ns)25 or high-quality
GaN single crystal (0.86 ns)26. Such a long lifetime indicates very
low density of non-radiative defects and negligible carrier trapping
to deep radiative defects, such as the green luminescence band
frequently seen in poor-crystallinity crystals and fi lms. Figure 1c
shows the temperature dependence of residual electron density
(n) and Hall mobility (μ) for an undoped ZnO fi lm. The value of
n is about 1 × 1016 cm–3 at room temperature and decreases with
decreasing temperature, with an activation energy of about 60 meV.
The value of μ is 300 cm2 V–1 s–1 and 5,000 cm2 V–1 s–1 at 300 K and
100 K, respectively, surpassing the best value for a ZnO bulk single
crystal27. Therefore, we conclude that ZnO fi lms grown at this high
temperature, Tg, on atomically smooth buffer layers can serve as an
arena for testing acceptor doping.
However, nitrogen, as one of the most promising acceptor
impurities, cannot be incorporated into ZnO at such a high Tg.
Nitrogen concentration (CN) decreases from a few times 1020 cm–3 at
Tg = 450 °C to a few times 1018 cm–3 at Tg = 700 °C, and at Tg = 950 °C
it is lower than the detection limit (mid-1017 cm–3)28. To solve this
dilemma, we have developed RTM to satisfy both high crystallinity
and high CN. We repeated a growth sequence in which a nitrogendoped
ZnO (ZnO:N) layer 10–15 nm thick is deposited at low
temperature (TL), followed by rapid ramp to high temperature (TH),
and growth of a 1-nm-thick layer at TH. Figure 2a shows an example
of the time variations of RTM. During the processes, the RHEED
pattern switched between two states as shown in the insets of Fig. 2b:
streaks (left) at TL and spots (right) at TH. The streak length, defi ned
by the full width at half maximum of streak intensity peak in the
vertical direction (shown on the insets), is plotted as a function of
time in Fig. 2b. During deposition at TL, the surface became gradually
rougher because of the low surface diffusivity of precursors.
On rapid ramping to TH, the surface smoothness recovered quickly.
Part of the time variation in Fig. 2b is magnifi ed in Fig. 2c (red)
together with the RHEED intensity oscillation (black). The two
oscillation patterns have opposite phase, indicating that initial TL
layers grown on atomically fl at TH layers grew in layer-by-layer mode
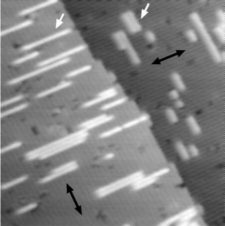
even at such a low temperature. Figure 2d shows an atomic force
microscopy image for a ZnO:N fi lm. The surface is composed of
atomically fl at, wide terraces and 0.26-nm-high islands. By using RTM, we
can grow ZnO:N fi lms with CN ranging from several times 1020 cm–3
to a few times 1018 cm–3 by tuning TL from 400 °C to 600 °C.
Among these fi lms, those grown at TL = 400 °C and TH = 950 °C
reproducibly showed p-type conduction. It is worth mentioning that
TH was chosen to satisfy the condition that hydrogen is completely
extracted from the fi lm29. Because residual hydrogen in the growth
chamber is incorporated in the fi lms during growth21, hightemperature
annealing may be essential not only for annihilating
non-equilibrium defects but also for removing hydrogen, if any, to
activate the acceptors.
The ZnO:N fi lms prepared by RTM have the same in-plane lattice
constant as that of the buffer layer. The out-of-plane lattice constant
is slightly expanded (+0.02%) compared with the bulk value.
The rocking curve width is as narrow as that of the undoped ZnO
fi lms, indicating that the structural quality of the p-type ZnO is very
high. The inset of Fig. 3 shows a set of raw data for the Hall resistance,
indicating p-type conduction as evidenced by the positive slope.
Figure 3 shows hole concentration as a function of temperature for
a p-type ZnO:N fi lm with CN = 2 × 1020 cm–3. From these data, we
can deduce an activation energy EA of 100 meV and compensation
ratio ND/NA ≈ 0.8. Here we note that ND/NA obtained in the present
p-type ZnO fi lm is higher than the reported value of 0.1 for p-type
ZnO grown by MBE9. Therefore, there is still room to increase hole
concentration by tuning the RTM growth condition.
The schematic structure of a typical homostructural p–i–n
junction is shown in Fig. 4a, which was grown throughout in layerby-
layer mode keeping an atomically fl at interface. Figure 4b shows
typical current–voltage characteristics. Fairly good rectifi cation
was obtained with a threshold voltage of about 7 V. The threshold
voltage is higher than the bandgap of ZnO (3.3 eV), mainly owing to
the high resistivity of the p-type ZnO layer. The electroluminescence
is measured by feeding in a direct current at room temperature.
This spectrum was measured from the top by detecting the light
escaping from the edge of the top electrode. The electroluminescence
spectrum shows luminescence from violet to green regions with
multi-refl ection interference fringes. We would expect exciton
emission at 3.2 eV from the undoped layer (i-ZnO) as shown in the
inset of Fig. 1b, but the electroluminescence spectrum apparently
shows a redshift. This is partly due to the low hole concentration in
p-type ZnO: electron injection from i-ZnO to p-type ZnO overcomes
hole injection from p-type ZnO to i-ZnO. The photoluminescence
spectrum (black) of a p-type ZnO fi lm is also shown. The higherenergy
side peak around 430 nm in the electroluminescence
spectrum matches well with the photoluminescence spectrum.
Another factor could be that the electroluminescence from active i-
ZnO is partly absorbed in the p-type layer because of a slight redshift
of the absorption edge for this layer. Nevertheless, the signal-tonoise
ratio of the electroluminescence spectrum shown in Fig. 4c is
much better than those reported previously11,30. More importantly,
the RTM technique established for reliable p-type doping of ZnO
enables us to improve the device performance further by optimizing
the growth parameters and device structures.
The next challenge will be to increase the hole concentration
by further optimizing the growth process of p-type ZnO.
Making a p-type (Mg,Zn)O fi lm is also an important challenge, not
only to prevent the majority of electrons from injecting into the
p-type layers, but also to avoid attenuation of band-edge emission
from the i-ZnO.
Figure 1 Thin fi lms of ZnO grown in persisting layer-by-layer mode show
high-quality optical and electronic properties. a, RHEED intensity oscillation
observed during ZnO thin-fi lm growth by laser MBE at a temperature of 950 °C on an
atomically smooth ZnO buffer layer formed on a ScAlMgO4 substrate. The oscillation
period corresponds to a 0.26-nm-thick charge-neutral molecular layer grown by
about 23 laser pulses. The oscillation persisted over fi lm growth to a thickness of
1 μm. The fi lm has the same lattice constants (a = 0.3250 nm, c = 0.5204 nm) as
the bulk values by relaxing the very small lattice mismatch of 0.09% with the SCAM
substrate in the high-temperature annealed ZnO buffer layer. The full-width at halfmaximum
of the rocking curve at the (002) refl ection is less than 18 arcsec, which
is close to the instrumental resolution. The growth direction is identifi ed, through
experiments similar to those reported previously32, to be [0001–] of the wurtzite
structure (oxygen face). b, Photoluminescence (PL) spectrum (inset) and temporal
decay of the luminescence intensity at the peak energy (indicated by triangle)
for an intrinsic ZnO fi lm. The dashed peak represents the temporal evolution of
the excitation laser pulse (Ti: sapphire, 242 nm, 30 mW, 80 fs). The lifetime of
free exciton emission exceeds 2.5 ns, indicating the high quality of the sample.
c, Temperature dependence of electron mobility and carrier concentration for an
undoped ZnO fi lm (solid circles) and a ZnO bulk single-crystal (open circles, after
Look et al.27). To ensure that the measurements extract the intrinsic properties of
the ZnO fi lm, a fairly thick fi lm (1 μm) was grown on a semi-insulating Mg0.15Zn0.85O
buffer layer which was annealed to prepare an atomically smooth surface before
the ZnO deposition23.
Figure 2 Atomically smooth ZnO fi lms doped with nitrogen can be grown by
a repeated temperature modulation technique. a, Temporal variation of growth
temperature, switching between TH and TL, during ZnO thin-fi lm growth. Layers
of ZnO:N with high nitrogen concentration (CN) were deposited at TL in the period
coloured blue. The layers were annealed and additional ZnO:N layers with low CN
were grown in the period coloured red in order to activate nitrogen as an acceptor
and recover surface smoothness, respectively. b, Typical RHEED patterns observed
during TL (left) and TH (right) periods are shown in the insets, representing rather
rough and atomically smooth surfaces by streaky and spotty patterns, respectively.
Temporal variation of streak length (defi ned by broken lines) is plotted as a measure
of the surface roughness. c, RHEED intensity (black) and streak length (red) are
plotted for the initial growth of high CN layer at TL as denoted by circle in b. Clear
oscillations having a half-phase shift confi rm the layer-by-layer growth mode.
d, An AFM image of the surface for a 500-nm-thick ZnO:N fi lm. The step height
corresponds to the thickness of a charge-neutral molecular layer of ZnO (0.26 nm).
Figure 3 Temperature dependence of hole concentration (p) in a p-type
ZnO doped with nitrogen. (Nitrogen concentration CN = 2 × 1020 cm–3.) A typical
variation of the Hall voltage during a magnetic fi eld scan is shown in the inset.
The hole mobility varies from 5 cm2 V–1 s–1 at 350 K to 8 cm2 V–1 s–1 at 300 K.
Activation energy EA and compensation ratio ND/NA are deduced to be 100 meV and
0.8, respectively, from the linear fi tting9 of p = (ND/NA – 1)(gA1/gA0)NvT3/2 exp(–EA/kBT),
where gA0 = 4 and gA1 = 1 are the unoccupied and occupied state degeneracies,
respectively, NvT3/2 = 2(2πmhkBT/h2)3/2 is the density of states in the valence band,
where kB is Boltzmann's constant and h denotes Planck's constant, and mh is
assumed to be 0.9m0 with m0 being free electron mass.
Figure 4 Zinc oxide homostructural p–i–n junction shows rectifying
current–voltage characteristics and electroluminescence (EL) in forward
bias at room-temperature. a, The structure of a typical p–i–n junction LED.
b, Current–voltage characteristics of a p–i–n junction. The inset has logarithmic scale
in current with F and R denoting forward and reverse bias conditions, respectively.
c, Electroluminescence spectrum from the p–i–n junction (blue) and
photoluminescence (PL) spectrum of a p-type ZnO fi lm measured at 300 K.
The p–i–n junction was operated by feeding in a direct current of 20 mA.
Nombre: Franklin J. Quintero
Asignatura: EES
Connect to the next generation of MSN Messenger
Get it now!